Clevor is ready to work when you — and your patients — need it.
It’s ready for immediate dosing. No complicated dose calculations or additional disposables, such as syringes or needles, required.
96% of users said the administration was easy or very easy in a study conducted with 100 dogs in the Clevor group.1
Convenient.
Clevor is available in a convenient, ready-to-use dropper that provides one treatment for a dog weighing 4 pounds to 220.5 pounds.
Each box of Clevor contains five, single-use droppers packed in individual aluminum foil pouches.
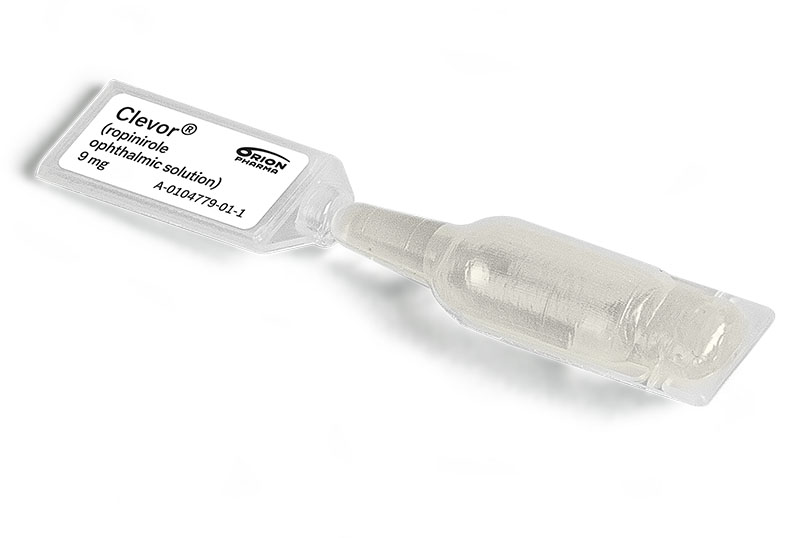
Accurate dosing that’s easy to administer.
Clevor should be administered by veterinary personnel.
For complete dosage and administration instructions, download the prescribing information.
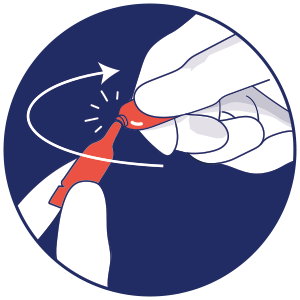
Opening the container
Wear gloves and protective eye wear when handling or administering this product to prevent accidental exposure.
Open the dropper by twisting off the tail.2

Administration
Keep the dog’s head steady in a slightly upright position.
Hold the dropper in an upright position without touching the eye.
Rest your finger on the forehead of your dog to maintain the distance between the dropper and the eye.
Squeeze the prescribed number of drops in to the eye(s).2
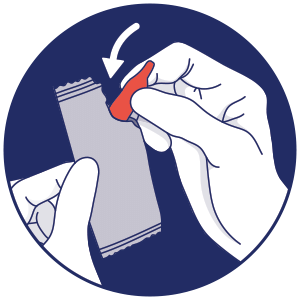
Storing the unused container
CLEVOR is a single use dropper and is light sensitive.
After administration, with gloves on, return the dropper to the aluminum pouch and place in the carton.2

Second dose
If the dog does not vomit, a second dose can be given 20 minutes after administration of the first dose.
This second dose is the same number of drops as the first dose.
Thirty minutes after opening, with gloves on, dispose of dropper, aluminum pouch, and carton.2
Refer to Animal Safety Warnings section of the full prescribing information for treatment of protracted vomiting.


References
- Suokko, M., Saloranta, L., Lamminen, T., Laine, T., Elliott, J. (2020) Ropinirole eye drops induce vomiting effectively in dogs: a randomised, double-blind, placebo-controlled clinical study. Veterinary Record, Mar 7;186(9):283.
- Clevor® (ropinirole ophthalmic solution) Prescribing Information. Orion Corporation.

