Quick to administer.
Quick to vomit.
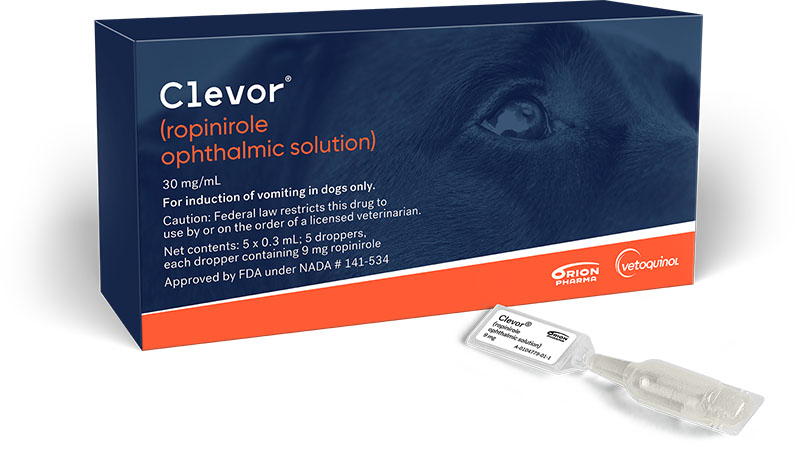
Clevor is the first and only FDA-approved product for emesis induction in dogs.
Its selective active ingredient and unique ocular administration make the process easy and injection-free — all of which add up to a quicker, less stressful process for you, your staff, the dog, and their owner.

Fast onset of action1
Selective active ingredient
Unique ocular administration


References
- Suokko, M., Saloranta, L., Lamminen, T., Laine, T., Elliott, J. (2020) Ropinirole eye drops induce vomiting effectively in dogs: a randomised, double-blind, placebo-controlled clinical study. Veterinary Record, Mar 7;186(9):283.



